Insight into All-Solid-State Batteries: How Does Sulfide Move Towards Mass Production?
Sulfide is recognized as one of the most potential technical routes leading to mass production at present, which has obviously become important in battery industry one of the positions.
As the only platform leading by Liyang municipal government, the transformation of intellectual property achievements of Institute of Physics of Chinese Academy of Sciences and the industrialization of sulfur-based all-solid-state battery technology, Zhongke Solid Energy has a self-evident position as an industry Vane.
How to move towards industrialization must be one of its important topics. This paper explores how Zhongke Solid Energy can solve a series of problems currently faced by sulfide all-solid-state batteries from the perspectives of patents, papers and official information.
Since the biggest change of solid state compared with liquid state is electrolyte, it is essentially to solve the problem of solid state electrolyte itself and its compatibility with other materials.
Sulfide all-solid-state battery problems mainly come from three dimensions: material, interface and cell.
At the material level, it mainly includes the electrochemical stability of the solid electrolyte itself and its stability in the air; The interface problem mainly refers to the compatibility between the solid electrolyte and the positive and negative electrode interface, as well as the solid interface in the process of ion migration, problems such as changes in volume structure; At the cell level, the thermal stability of sulfide solid-state batteries, performance attenuation caused by volume changes, mass production and cost are all difficulties in industrialization.
01.
Ionic conductivity
andLiquid electrolyte is different, solid electrolyte ion migration energy barrier (the minimum energy contained in activated molecules in chemical reaction that can participate in chemical reaction) is high (more than 10 times of liquid), therefore, the ionic conductivity is low.
At present, there are four main ways to improve the ionic conductivity of solid-state batteries:
all in all, improving the ionic conductivity of solid-state batteries is nothing more than increasing the ion concentration of solid-state batteries, and then reducing the obstacles of lithium ions in various bulk phases, grain boundaries, interfaces and other positions during their migration.
There are four main mechanisms for improving the ionic conductivity of solid electrolyte by doping ideas:
a) adjust the ion occupancy rate of Li + through different dopant elements and dopant content to make the ion conduction faster;
b) adjust the lithium vacancy concentration through ion doping to change the migration mechanism of lithium ions;
c) charge compensation through ion doping increases Li + content and Li + migration number in electrolyte;
d) local disorder caused by isovalent ion doping enhances the ionic conductivity of Li conductive material.
 </p>
</p>
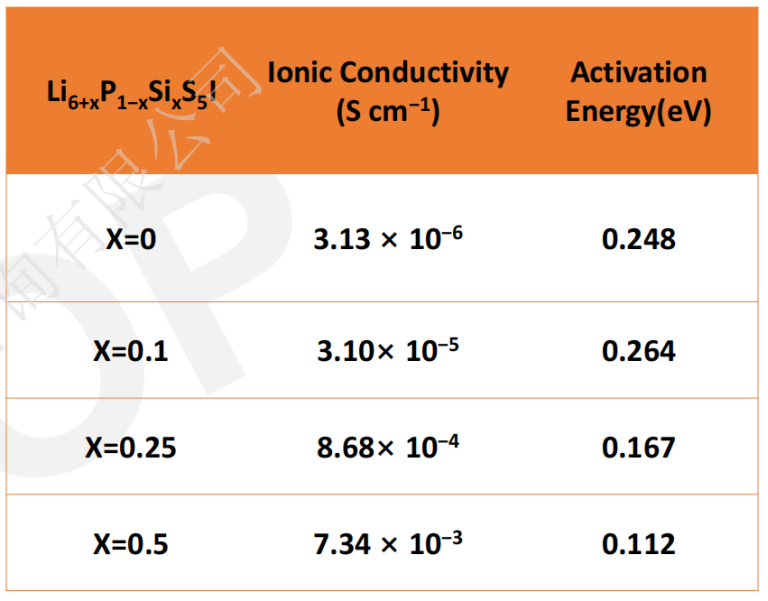
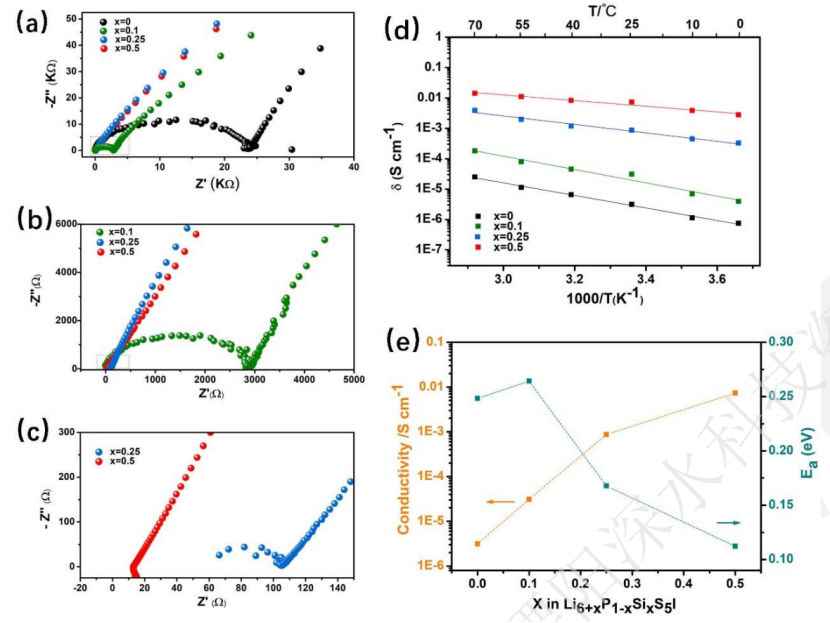
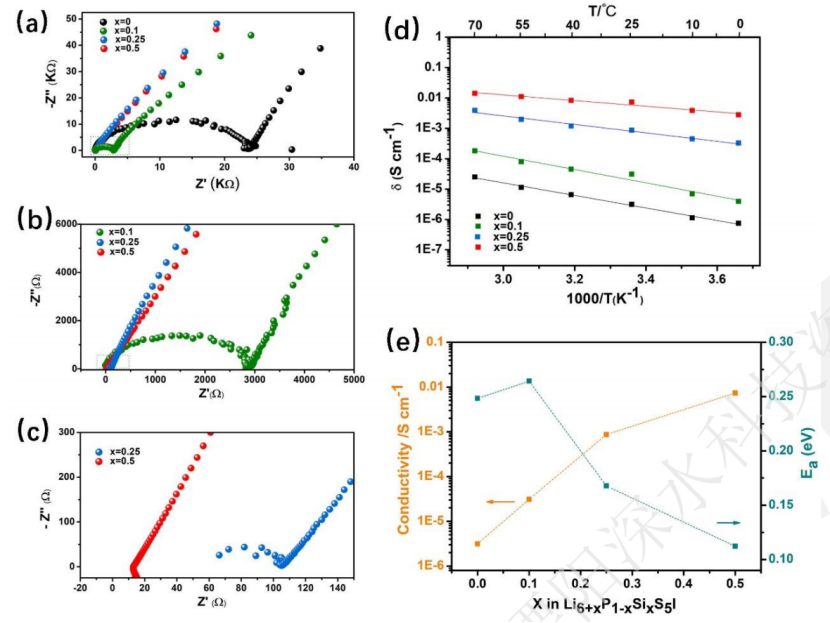
Zhongke Solid Energy uses two of these mechanisms (a + d) to improve the ionic conductivity. One is to use Si4 + ion-doped Li6 + xP1-xSixS5I (0≤x≤0.5) to increase the content of Li + to promote the diffusion of Li + in the substructure, and the second is that Si4 + on the P5 + bit increases I? /S2? The disorder of position changes the substructure of lithium and promotes the migration of Li +, resulting in the increase of ionic conductivity and the sharp decrease of activation energy.
02.
Solid interface and lithium Crystal problem
the problems of poor contact between solid electrolyte and electrode interface, the growth of lithium crystals on the negative electrode side, and the need for tens to hundreds of MPa pressure to maintain good contact at the interface during the operation of sulfide solid battery, all limit the performance and improvement.
In view of the above problems, the current solution given by Zhongke solid energy is introduce new materials and new processes, or optimize the processing on the original materials.
At the material level, the Wu Fan team of the Institute of Physics of Chinese Academy of Sciences and Zhongke guanneng developed a new type of room temperature liquid lithium anode material 3D LiSi @ Li-Phen-Ether (3D LSLL), which is fully infiltrated by the lithium-friendly Li-Phen-EtherLiSi alloy powder forms 3D Li +/e-fast migration path to improve interface contact, inhibit nucleating/growth of lithium branches, and improve specific capacity and cycle performance.
At the same time, Wu Fan's team also developed an ionic conductivity and electronic conductivity soft carbon(SC )-Li3N interface Layer, its in-situ Lithiation Reaction can not only convert SC lithium into LiC6 with good electron/ion conductivity, the mixed phase Li3N is also successfully converted into pure phase β-Li3N with high ionic conductivity/ionic diffusion coefficient and lithium metal stability. The mixed conductive interface layer is conducive to the rapid transmission of Li + at the interface and induces the uniform deposition of metal lithium inside it, which effectively inhibits the formation of lithium crystals and improves the performance of ASSLMBs.
In the new process, Zhongke Solid Energy proposed a kind solid phase passivation, lithium metal protection is carried out on lithium metal solid-state battery with artificial solid-state electrolyte interface (SEI).
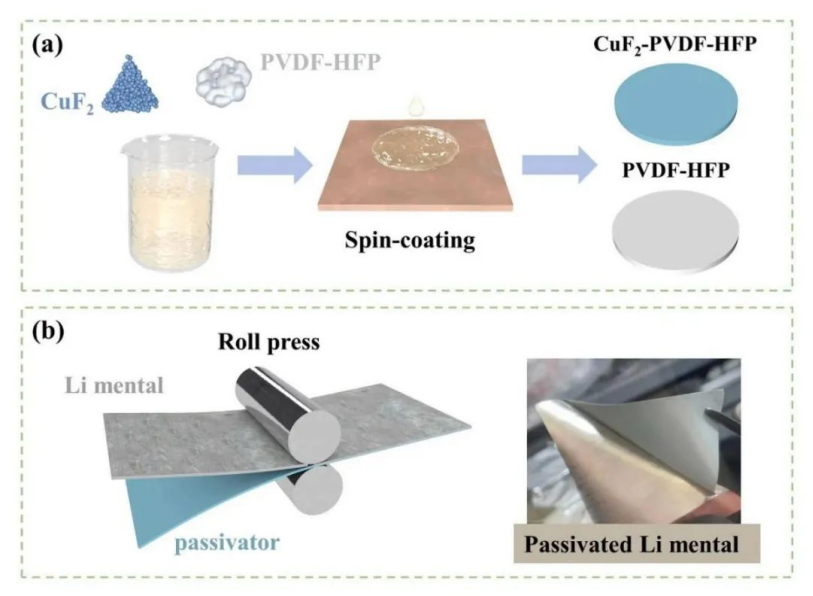
Schematic diagram of in-situ solid-phase passivation:(a) preparation process of passivation film; (B) In-situ solid-phase passivation process
the prepared passivation film is covered on the surface of lithium metal, and the passivator molecules are contacted and interacted with lithium metal closed-up surface by high temperature rolling. Under the driving of hot pressing, the passivator molecule undergoes solid-solid bonding reaction with lithium atom. After a specific treatment time, the substrate of the passivation film is separated, reacted with lithium metal, adjusted the surface composition of lithium, passivated the surface of lithium metal, thus inhibiting the growth of the branches.
At the positive interface, Zhongke Solid Energy Introduces DOL in-situ polymerization gel interface layer, realize the positive lithium iron phosphate and the 3D LSLL negative sulfide solid-state battery developed by it realize over 300 cycles under the conditions of 0.5 MPa external pressure, room temperature environment and 2C magnification.
Aiming at the interface problem, Zhongke Solid Energy is also solved by means of surface vulcanization of ternary positive electrode material, sulfide solid electrolyte + liquid lithium negative electrode, etc.
03.
Air stability
sulfide electrolyte exposure in the air will produce toxic gas H2S, electrolyte structure damage, electrochemical performance attenuation, so its stability in the air is very poor. In the process of synthesis, storage, transportation and post-treatment, it relies heavily on inert gas or drying chamber, which will greatly increase the corresponding cost.
The main way for Zhongke Solid Energy to develop air-stable sulfide electrolyte is to apply h2s-absorbent, Element substitution, new material design, surface engineering and sulfide-polymer composite electrolyte.
Currently, the industry is pushing dry Process Technology, in the process of making the film, organic and polar solvents will be avoided, and only a small amount of adhesive is needed, which can cut off the risk of toxic gases generated by reaction with the solvent from the root. At the same time, dry technology saves solvent, solvent evaporation, recovery and drying equipment, which will reduce part of the cost to a certain extent. This is also the key technology currently laid out by Zhongke solid energy to solve the problem of air stability during the preparation of sulfide electrolyte.
One-Step gas phase method the sulfide electrolyte is synthesized to maintain its air stability during the preparation process. Compared with the traditional solid-liquid phase synthesis method in the preparation of sulfide electrolyte, the transition depends on glove box argon atmosphere protection, low yield, high raw materials, etc.
One-Step gas phase method is mainly taking the gasification CS2 as vulcanising preparations, sulfides the oxide raw materials with low cost and stable air, and the optimized sulfide electrolyte(LSAS does not react with water, but absorbs H2O molecules to form hydrate containing 13 crystal water) the ionic conductivity is greatly improved and has good air stability. Without glove box in the preparation process, a large amount of sulfide electrolyte can be obtained from tube furnace and a series of air-stable sulfide electrolyte can be synthesized in the air environment.
Reference patents (some patents are other personnel or achievements of the Institute of Physics of Chinese Academy of Sciences) and related as follows:
1. Pushun Lu, Lulu Li, Shuo Wang, Jieru Xu, Jian Peng, Wenlin Yan, Qiuchen Wang, Hong Li, Liquan Chen, Fan Wu *. Superior all-solid-state batteries enabled by gas-phase synthesized sulfide electrolyte with ultra-high moisture stability and ionic conductivity. Adv. Mater. 2021, DOI: 10.1002/adma.202100921
2. Hard-carbon-stabilized Li-Sianodesfor high-performance all-solid-statei-Lion batteries,Nature Energy (IF=67.439)
3. Air Stability of Solid-State Sulfide Batteries and Electrolytes
4. Doping Strategy and Mechanism for Oxide and Sulfide Solid Electrolytes with High Ionic Conductivity,Journal of Materials Chemistry A
5. Liquid-phase synthesis of Li2S and Li3PS4 with lithium-based organic solutions,Chinese Physics B
6. Jiacheng Wang, liquid Chen, Hong Li, and Fan Wu *. Anode Interfacial Issues in Solid-State Li Batteries: Mechanistic Understanding and Mitigating Strategies
7. Low-pressure dendrite-free sulfide solid-state battery with 3DLiSi @ Li-Phen-Ether anode
8. Dendrite-Free All-Solid-State Lithium-Metal Battery By In-situ Phase Transformation of Soft Carbon-Li3N Interface Layer
9. Wide-temperature, Long-cycling, and High-loading Pyrite All-solid-state Batteries Enabled by Argyrodite Thioarsenate Superionic
10. Superior Low-Temperature All-Solid-StateBattery Enabled by High-lonic-Conductivityand Low-Energy-Barrier interface
11. Dendrite-free lithium-metal all-solid-state batteries by solid-phase passivation
12. Stable Interface Between Sulfide Solid Electrolyte and-Room-Temperature Liquid Lithium Anode
13. Anode Interfacial Issues in Solid-State Li Batteries: Mechanistic Understanding and Mitigating Strategies
14. Thermal Stability between Sulfide Solid Electrolytes and Oxide Cathode
15. Superior all-solid-state batteries enabled by gas-phase synthesized sulfide electrolyte with ultra-high moisture stability and ionic conductivity
 Dongguan Juneng New Energy Technology Co., Ltd.
Dongguan Juneng New Energy Technology Co., Ltd.
 137 5142 6524(Miss Gao)
137 5142 6524(Miss Gao)
 susiegao@power-ing.com
susiegao@power-ing.com
 Xinghuiyuan High tech Industrial Park, Dalang Town, Dongguan City, Guangdong Province
Xinghuiyuan High tech Industrial Park, Dalang Town, Dongguan City, Guangdong Province













 Yue Gong Wang An Bei No. 4419002007491
Yue Gong Wang An Bei No. 4419002007491